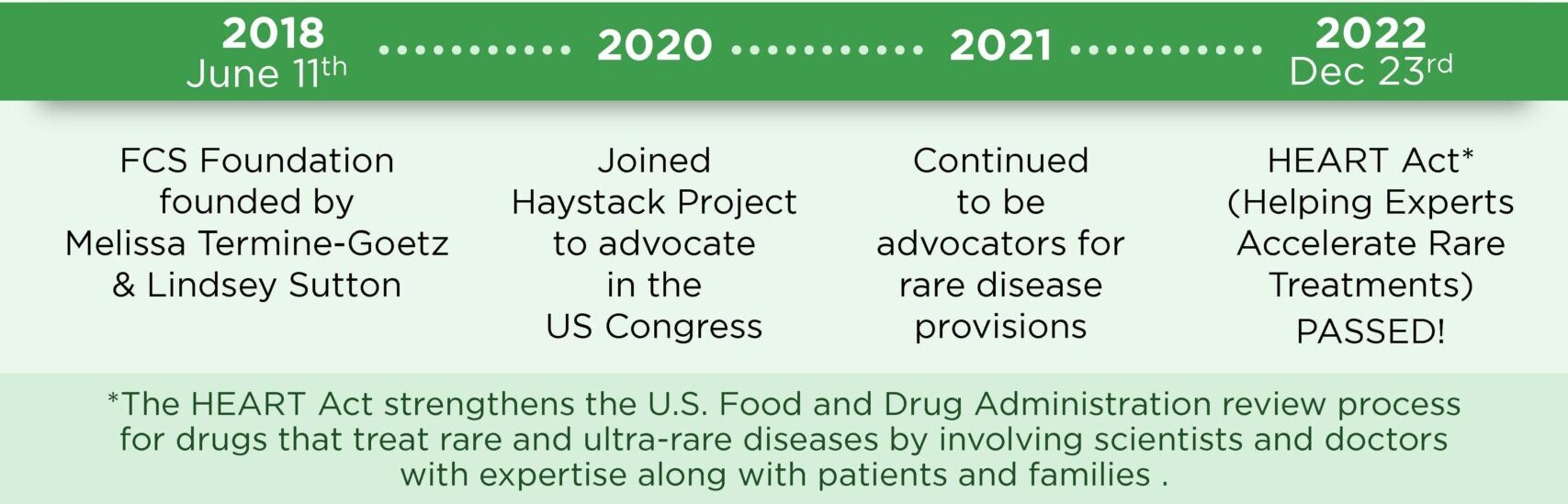
The Helping Experts Accelerate Rare Treatments (HEART) Act was passed in the Omnibus on December 23, 2022. This critical legislation creates a program to support and promote research, improve the quality of life for those living with FCS and other rare diseases, and helps ensure that patients have access to life-saving treatments.
The HEART Act was introduced by Representatives Paul Tonko (D-NY) and David McKinley (R-WV) in the House and Senators Bob Casey (D-PA) and Tim Scott (R-SC) in the Senate.
Tonko was inspired to introduce this legislation through his work with Melissa Termine Goetz, constituent and Co-President of The Familial Chylomicronemia Syndrome (FCS) Foundation, whose daughter is living with FCS.
“As patients, we want to feel confident that the division reviewing our application has the needed experience in rare diseases,” noted Goetz.


- Requires a study on sufficiency and use of FDA mechanisms to incorporate patient/clinician perspective in FDA processes related to applications for drugs for rare diseases & conditions
- Calls on the FDA to be required to develop an annual report on progress of rare disease drug applications
- Requires FDA host a public meeting to address approaches to increasing and improving engagement with rare disease or condition patients, groups representing such patients, rare disease or condition experts, and experts on small population studies, in order to improve the understanding with respect to rare diseases or conditions in terms of patient burdens, treatment options and side effects
- Directs a review of the European Union’s best practices for approving rare disease drugs

For all HEART Act specifics, please visit:
S. 4348 — 117th Congress: FDASLA Act of 2022.” www.GovTrack.us. 2022. December 23, 2022

